Written by Saachi Talwai – Senior Digital Engineer
In life sciences, speed and rigor often pull in opposite directions, especially in early-stage studies where data is sparse, stakes are high, and every decision must be defensible. At Sonata Software, we bring these forces together with an AI-agentic approach that pairs statistical depth with clinical context.
This blog demonstrates how two purpose-built agents, TrialAnalyzer and InsightAgent, work in tandem to turn a small, simulated hypertension trial into actionable guidance in minutes, not days. You’ll see how secure tool connections, a clear agent-to-agent (A2A) handoff, and domain-aware reasoning produce balanced recommendations that weigh efficacy against safety, exactly what clinical and regulatory teams need to move forward with confidence. (All data shown here is simulated and for educational purposes.)
The challenge
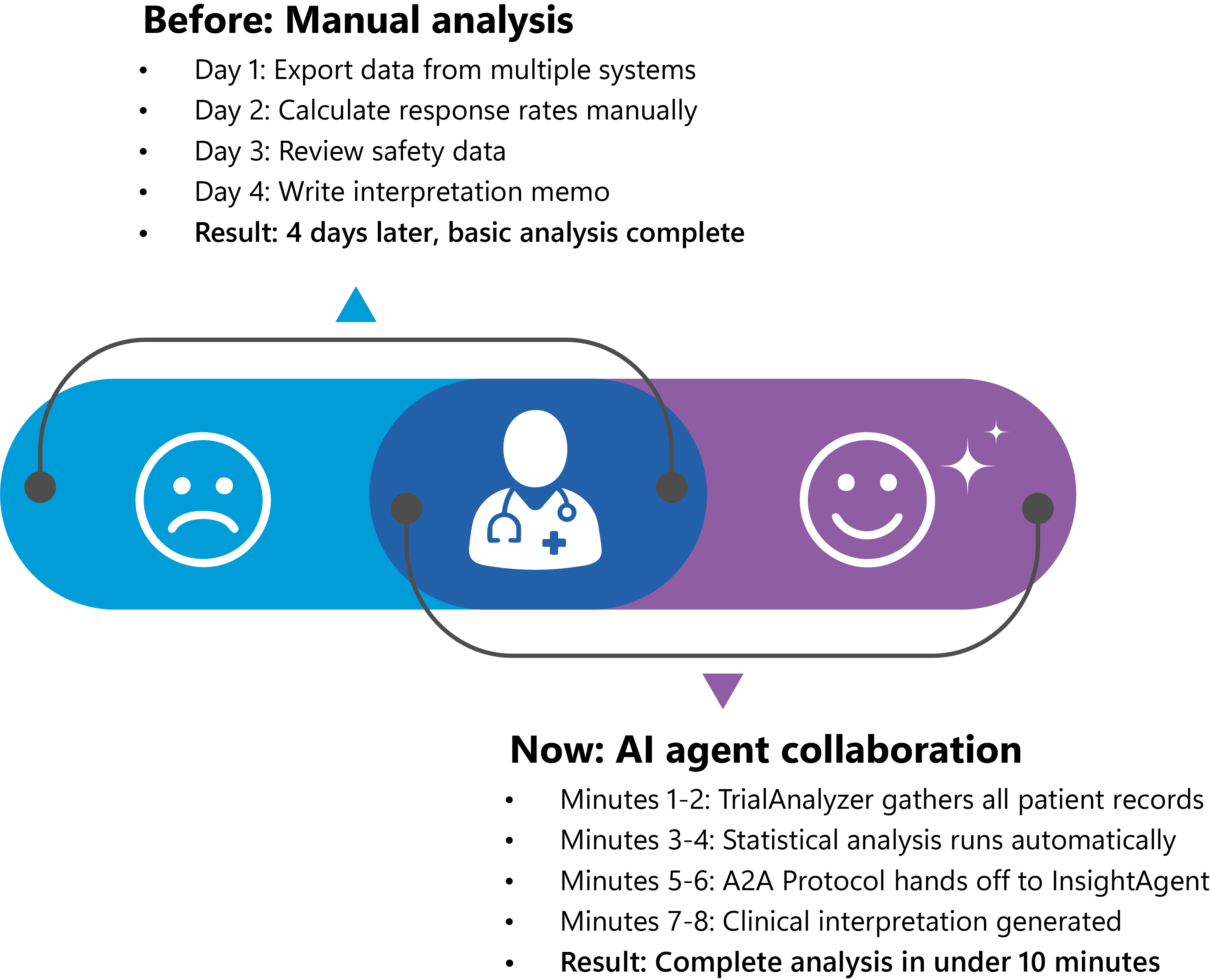
Dr. Martinez, a clinical researcher, has a small dataset from a new hypertension trial and needs to understand what the early data is telling her. In the past, even analyzing this limited dataset would take days of manual work across different systems.
Important note: This demonstration uses a small sample clinical trial dataset (10 patients per trial) to illustrate how AI agents collaborate. All patient data and results are simulated for educational purposes.
The solution: Two AI agents working together
Meet the team

TrialAnalyzer Agent - the data detective
- Lives for numbers and patterns
- Never misses a detail, regardless of dataset size
- Can process any amount of data consistently
InsightAgent - the clinical strategist
- Translates medical findings into actionable decisions
- Understands what early data means for future research
- Knows when sample sizes limit conclusions
How they collaborate
- Step 1: Dr. Martinez makes a request
She types: "Analyze our HTN001 trial data"
- Step 2: TrialAnalyzer springs into action
Connecting to specialized tools:
Clinical trial MCP server: "Give me everything on HTN001"
- retrieve_trial() pulls basic trial info
- get_patient_data() retrieves all patient records
- list_trial() lists available trials
- store_trial() stores single trial json
Analytics MCP server: "Now let's crunch these numbers"
- analyze_safety() reviews adverse events
- compare_groups() looks at treatment vs control groups
- evaluate_endpoints() measures blood pressure reduction
- analyze_demographics() analyze trial demographics
What TrialAnalyzer discovers:
Trial overview
- Trial Name:Hypertension Management Study
- Phase: Phase III
- Indication: Essential Hypertension
- Status: Active
- Patient Count: 10
Demographic analysis
- Mean Age: 64.2 years
- Gender distribution: Male: 52%, Female: 48%
- Ethnicity breakdown: Caucasian: 68%, Hispanic: 18%, African American: 14%
- Demographic balance score: 85.0 (out of 100)
Endpoint evaluation
Primary endpoint: Reduction in systolic BP ≥ 20mmHg
- Achieved: Yes
- Efficacy rate: 78.5%
- Mean effect size: 22.3 mmHg reduction
- Statistical significance: p-value: 0.0023 (Significant)
- Clinical significance: Yes
Safety monitoring
- Total adverse events: 23
- Adverse event rate: 230.0%
- Serious adverse events: 2
- Serious AE rate: 20.0%
- Safety risk level: High
- Safety concerns: Serious adverse event rate exceeds acceptable threshold
Group Comparison Analysis
- Treatment group: 6 patients, 78.5% response rate
- Control group: 4 patients, 32.1% response rate
- Absolute risk reduction: 46.4%
- Number needed to treat: 2.2
- Effect size: 1.784 (large)
- Step 3: The smart handoff
TrialAnalyzer uses the A2A Protocol to send a structured message to InsightAgent:
"HTN001 analysis complete. Excellent efficacy demonstrated (78.5% response rate, 22.3 mmHg reduction). However, significant safety concerns identified: 230% adverse event rate, 20% serious AE rate. Strong statistical significance but high safety risk requires immediate attention. Ready for clinical interpretation and business decision."
- Step 4: InsightAgent provides clinical wisdom
Connecting to specialized tools:
InsightAgent receives this handoff and applies business strategy:
Decision: CONDITIONAL
Confidence level: 90.0%
Rationale:
- Efficacy: Strong results with significant blood pressure reduction
- Safety: High adverse event rate requires enhanced safety measures
- Statistical analysis: Large clinically meaningful difference between groups
Risk Assessment:
- High safety risk: Due to high rate of adverse events and serious adverse events
- Regulatory risk: Potential delays and additional requirements from regulatory bodies
Recommendations:
- Enhanced safety: monitoring: Implement robust safety monitoring protocol
- Dose adjustment: Consider dose reduction or modified dosing schedules
- Regulatory engagement: Prepare for discussions with regulatory bodies
- DSMB review: Establish Data Safety Monitoring Board review schedule
- Step 5: Dr. Martinez gets her answer
Trial: HTN001 (Hypertension Management Study)
Findings: Excellent efficacy (78.5% response, 22.3 mmHg reduction) but high safety concerns
Decision: CONDITIONAL - Address safety before proceeding
Next Steps: Implement enhanced safety monitoring, dose evaluation
Regulatory: Prepare FDA communication plan for safety mitigation
The magic of working together
What makes this special
Smart Data Awareness
Both agents understand how to interpret findings appropriately based on dataset size.
Contextual Communication
The A2A Protocol ensures InsightAgent knows exactly what TrialAnalyzer found and why it matters.

Balanced Assessment
The system identifies both strong efficacy and safety concerns, providing nuanced recommendations rather than simple go/no-go decisions.
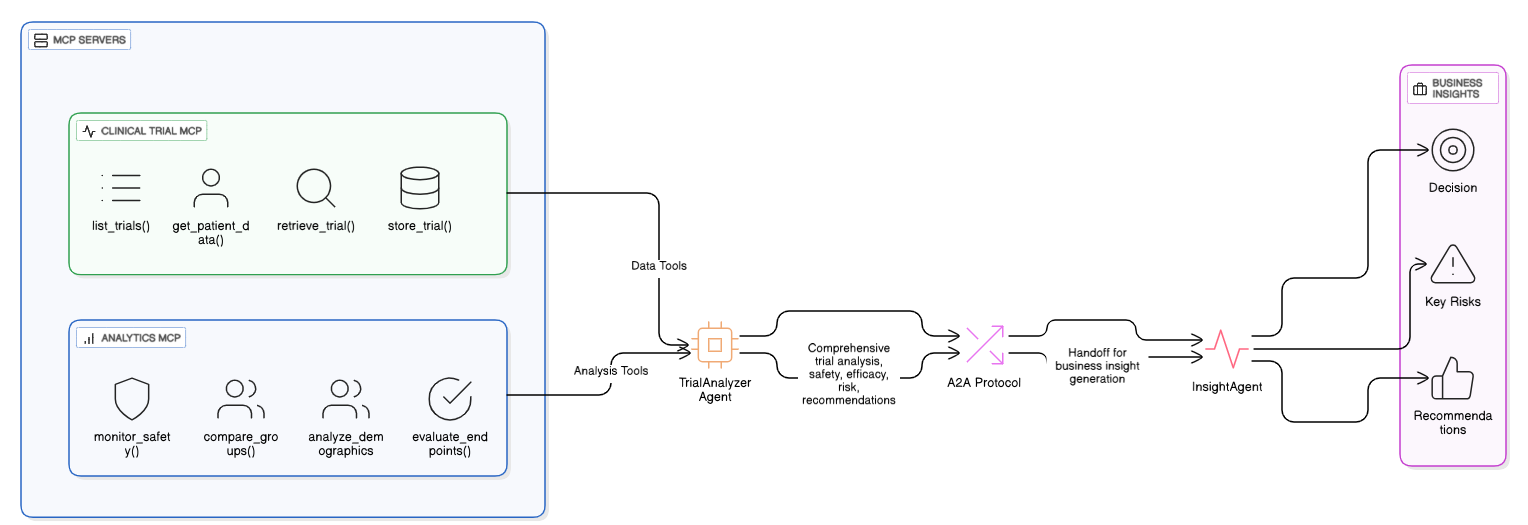
The architecture in action
1. Data tools (MCP Servers) provide specialized access to trial information
2. TrialAnalyzer orchestrates data gathering and comprehensive analysis
3. A2A protocol enables seamless handoff with full context
4. InsightAgent applies clinical judgment and business strategy
5. Dr. Martinez gets actionable insights with clear next steps
Real Impact
Dr. Martinez now gets immediate, comprehensive feedback that balances efficacy results with safety concerns. She can make informed decisions about trial modifications, regulatory strategy, and risk mitigation—all based on proper interpretation of both promising results and limiting factors.
The key insight: Two AI agents working together provide more reliable analysis than manual methods, delivering nuanced decisions that account for multiple factors simultaneously.
Conclusion
Clinical decisions demand more than fast numbers, they demand trustworthy judgment. By combining a data-obsessed TrialAnalyzer with a clinically savvy InsightAgent, this architecture delivers exactly that: rapid, reproducible analysis with the nuance to flag risks, justify next steps, and prepare for regulatory dialogue. The result is a tighter loop between researchers, safety oversight, and strategy. so promising signals are advanced responsibly and issues are surfaced early. At Sonata Software, our AI Engineering teams help life-sciences organizations design and deploy agentic workflows like this across R&D and pharmacovigilance, built with enterprise-grade security, governance, and human-in-the-loop controls.
Ready to explore the approach for your trials?
Try it yourself: Clinical Trial Analysis
Move faster without compromise. Agentic AI turns days of manual work into minutes of trustworthy, regulatory-ready insight.